Contact:
4008465777
Tonghua Dongbao's insulin products win the bid for centralized procurement, increasing the company's market share
On November 26, 2021, China's Joint Procurement Office (JPO) released the preliminary results of the sixth round of volume-based procurement (VBP) tender for insulin. Tonghua Dongbao's insulin products won the bid as Category B candidates, which conforms to the management's expectation and its business plan. Benefiting from the diverse product portfolio and economies of scale, the Company can further increase its market share while earning a steady stream of profits, which lays a solid foundation for its vision of becoming an explorer and leader of novel drug R&D in the field of endocrinology.
The VBP winning drugs include Human Insulin Injection, Insulin Glargine Injection, and Insulin Aspart Injection with different specifications. The figure below shows the tender prices (prices of insulin, available in 3 mL/300 IU cartridges) and the agreed purchase quantity of each drug.
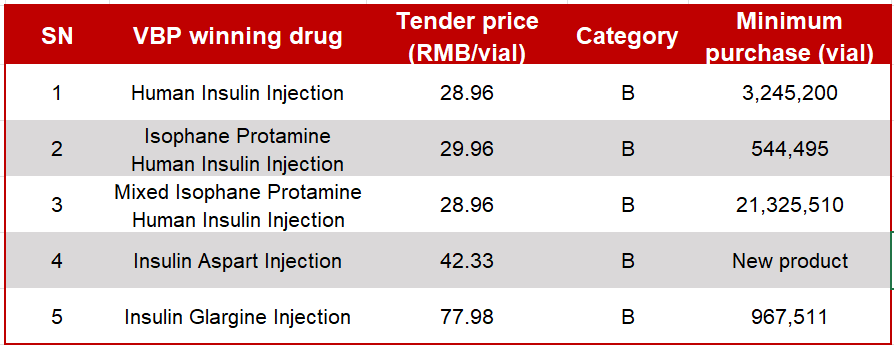
The Company's insulin analogs have delivered an impressive performance in the tender. Insulin Glargine Injection and Insulin Aspart Injection won the bid as Category B candidates. This will accelerate the availability of the two drugs, which are still at the early stage of release, across China, boost their sales, and dramatically increase the market share within a short time. As a long-standing player in China's diabetes therapeutics market, Tonghua Dongbao boasts extensive expertise and excellent after-sales services. Winning the bid, the Company will make a full foray into the insulin analog market, to speed up the development of homegrown insulin products and create new profit growth points.
Tonghua Dongbao's human insulin has also achieved a good result. Currently, the Company has a 38% share of China's human insulin market. With its human insulin products winning the bid as Category B candidates, the Company will be able to further boost its product sales, increase its market share and insulin's market penetration, and accelerate the substitution of domestic insulin products for foreign ones.
Tonghua Dongbao has also accelerated innovation in the therapies of diabetes and other endocrine diseases and doubled down on novel drug development. This year, the Company has unveiled three Class 1 novel diabetes drugs and the R&D projects of two Class 1 novel gout drugs. Among them, the world's first SGLT1/SGLT2/DPP4 triple inhibitor was approved for clinical trials in June 2021, and the clinical trial application of URAT1 inhibitor, a Class 1 novel drug for gout (hyperuricemia), was accepted in October 2021. Meanwhile, BC Lispro was approved for parallel phase III and phase I clinical trials. These milestones are the preliminary results of Tonghua Dongbao's efforts in R&D and talent training.
"The volume-based procurement program benefits both the country and the people. With all our insulin products enrolled in the program, we fulfill our social responsibility. The program presents both a challenge and an opportunity for enterprises. We believe that it will drive the high-quality development of enterprises in the long run, accelerate the availability of homegrown insulin products, and drive business innovation and transformation. As a leading insulin company in China, Tonghua Dongbao boasts a comprehensive R&D pipeline in the field of diabetes and other endocrine diseases. Winning the bid has laid a foundation for the future development of the Company. As its research projects are commercialized and transformation strategy is implemented, Tonghua Dongbao will make a new leap," said Leng Chunsheng, Chairman of Tonghua Dongbao.










